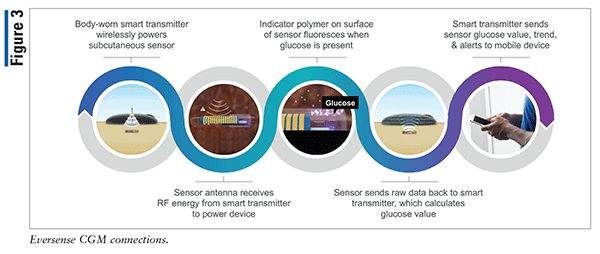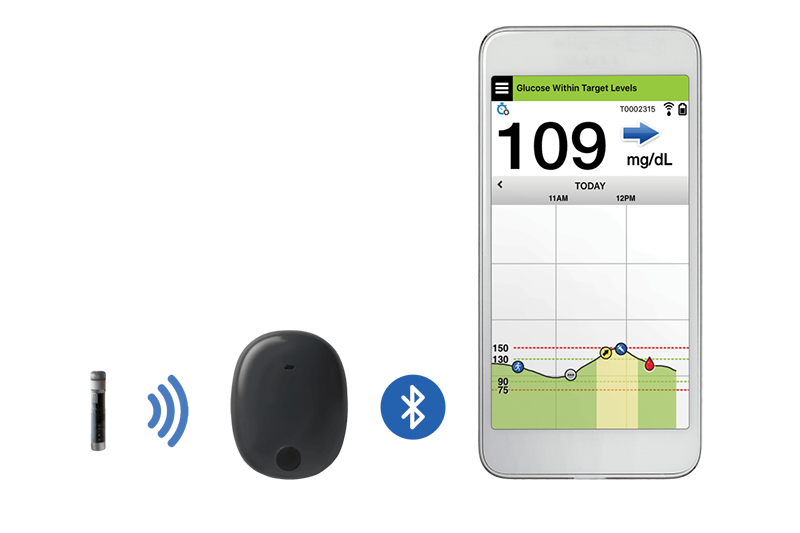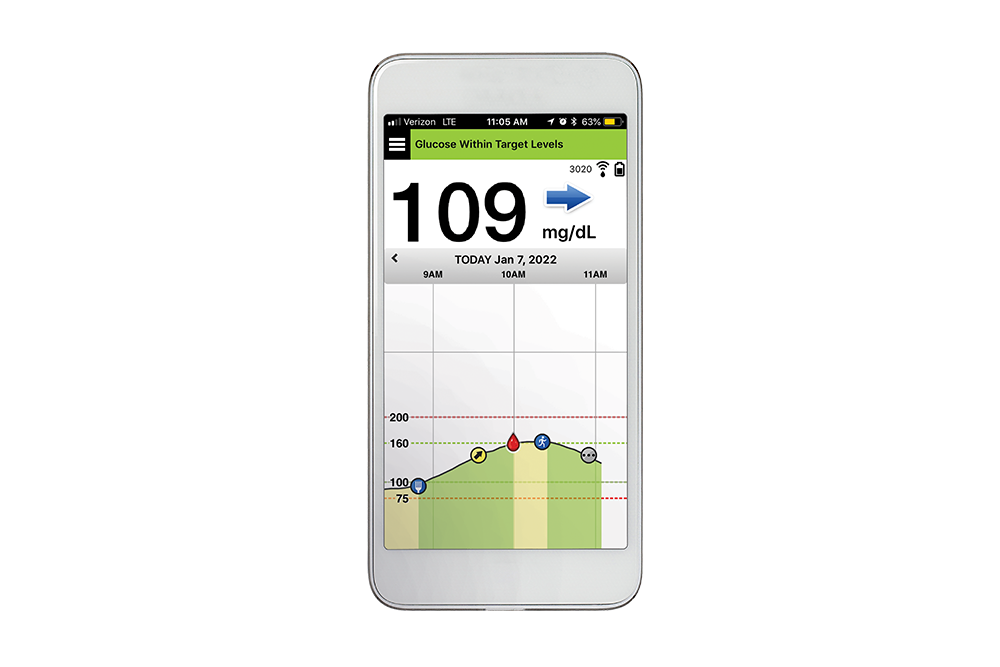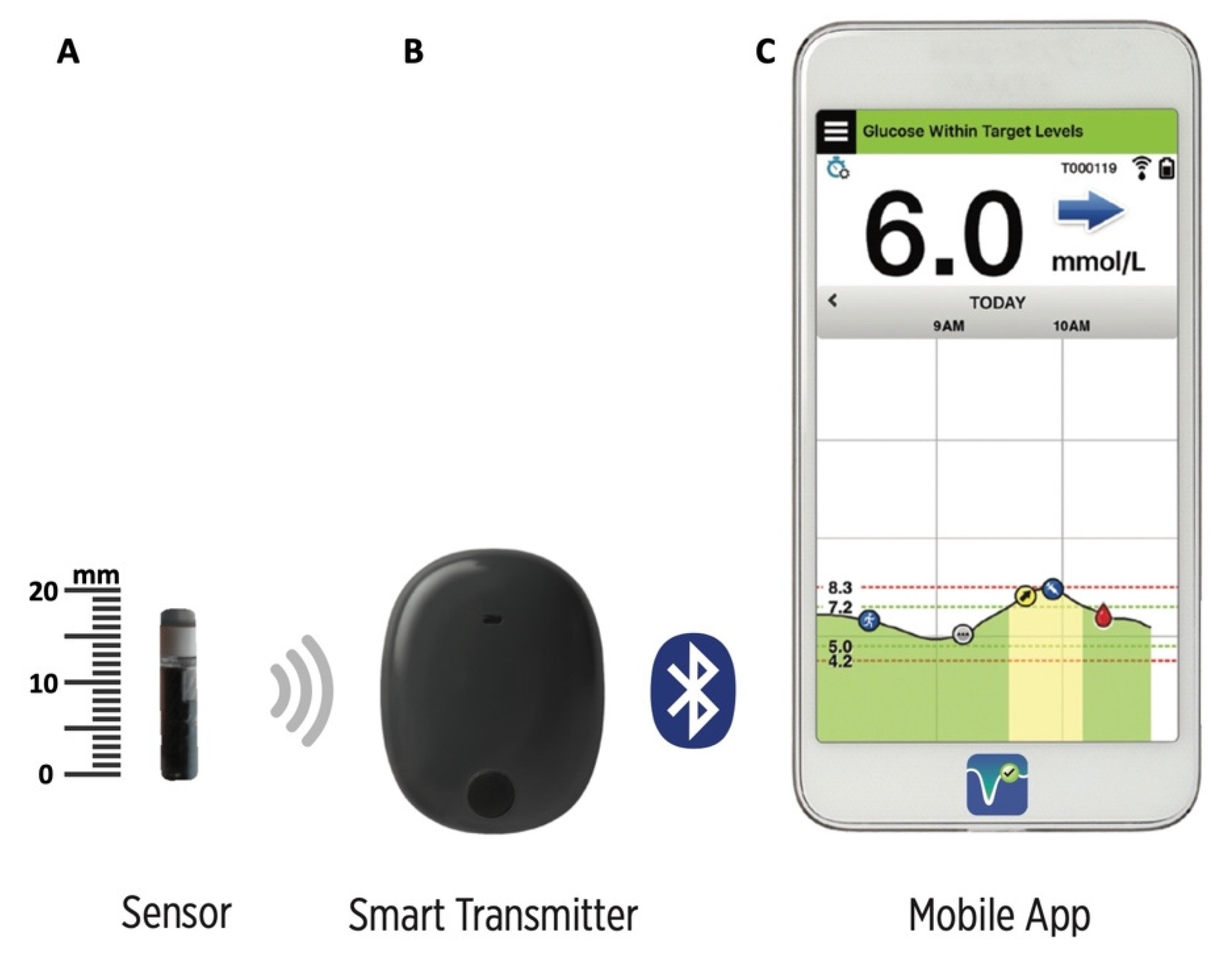
Animals | Free Full-Text | Clinical Use of a 180-Day Implantable Glucose Monitoring System in Dogs with Diabetes Mellitus: A Case Series

Eversense sensor placement. (a) The positioning guide for the Eversense... | Download Scientific Diagram