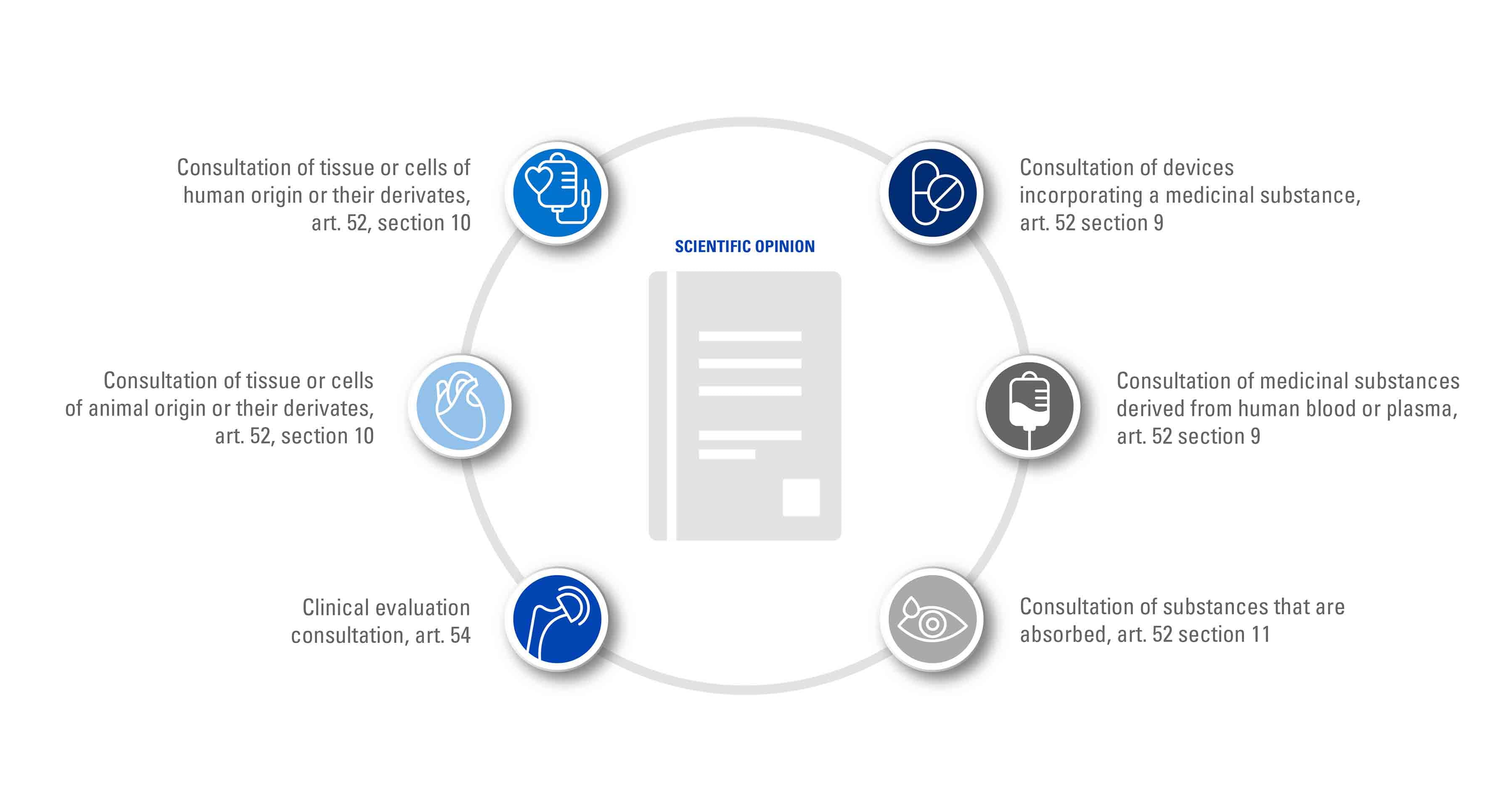
Europe - MDCG 2021-22 : Clarification on “first certification for that type of device” and corresponding procedures to be followed by notified bodies, in context of the consultation of the expert panel

Mario Gabrielli Cossellu on LinkedIn: #mdcg #actions #notifiedbody #manufacturers #transition #regulations #mdr…

EU IVDR News! (EUDAMED) Guidance on harmonised administrative practices and alternative technical solutions until Eudamed is fully functional - Formiventos

Meditrial - 📢 #EU: The European Commission Medical Device Coordination Group (#MDCG) answers questions on #clinicalinvestigation for medical devices under the upcoming #MDR. Read more: https://ec.europa.eu/health/sites/health/files/md_sector/docs ...
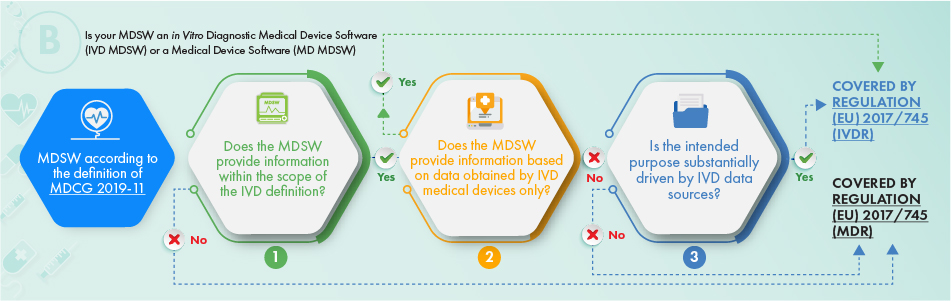
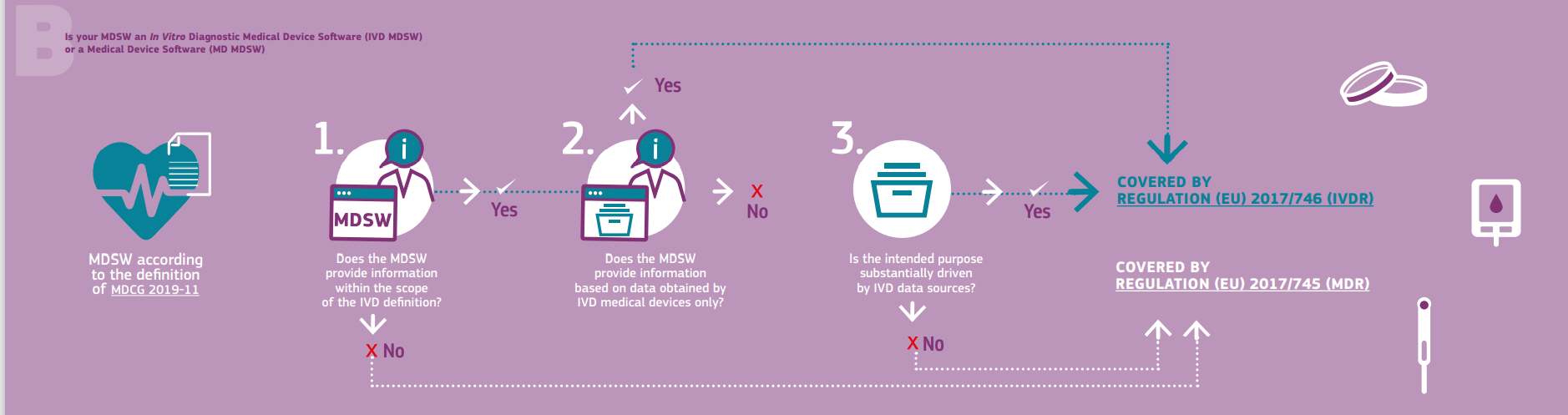
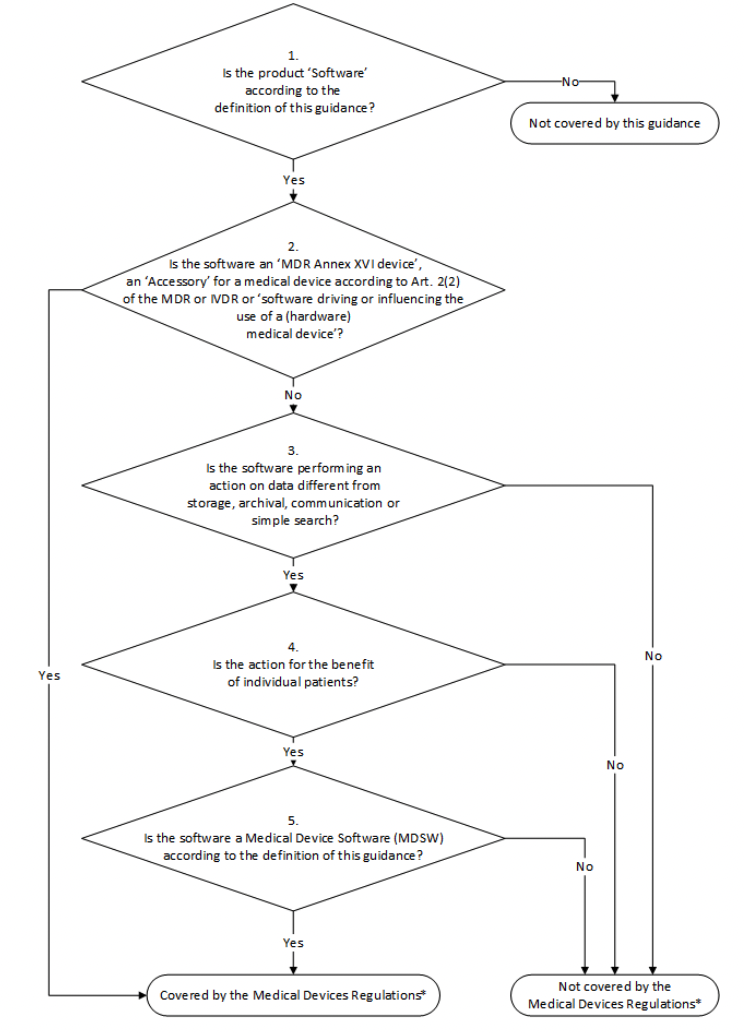
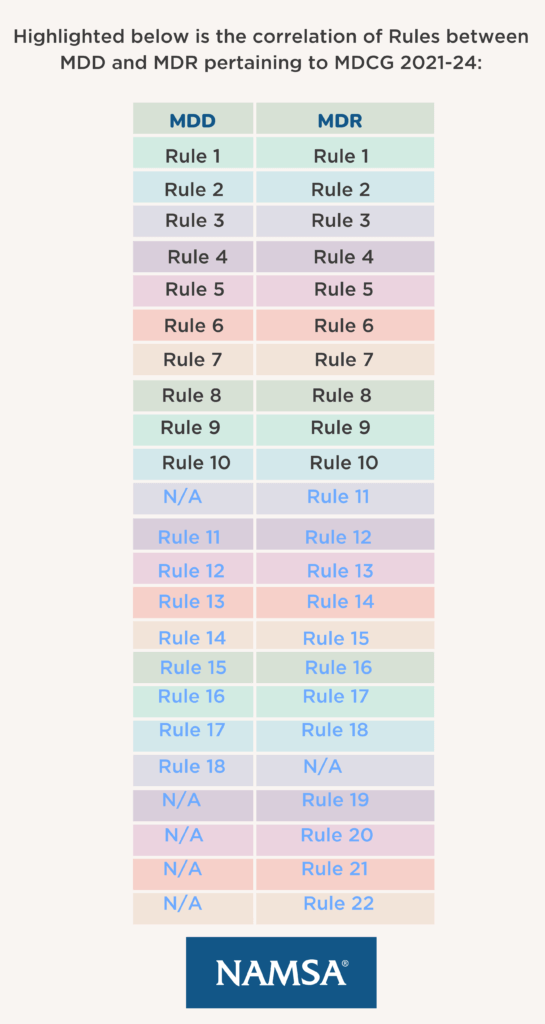
MDCG Guidance for Medical Device Software | Freyr - Global Regulatory Solutions and Services Company